SCIENTISTS FROM BRNO HAVE REVEALED THE STRUCTURE OF THE BRIGHTEST ENZYME. THEY CAN MAKE IT GLOW EVEN MORE INTENSELY AND INCORPORATE IT INTO HUMAN CELLS
Shrimp luciferase is the brightest. And now we know how it works.
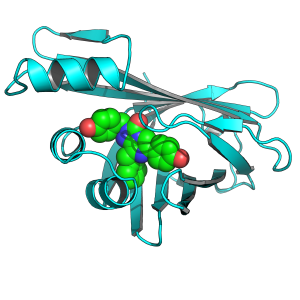
Scientists worldwide have been attempting to uncover the essence of this enzyme for decades, but it was only recently that researchers from Brno succeeded. They isolated one of the smallest yet brightest enzyme celebrities, called nanoluciferase, from the deep-sea shrimp Oplophorus gracilirostris.
When a predatory fish develops a taste for this shrimp, it, as a defensive mechanism, releases a highly viscous secretion into its surroundings, which soon transforms into a stunning light show. While the predator is baffled by this underwater fireworks display, the shrimp gains valuable time to escape.

Shrimp defends against dragonfish
Oceanographer Edith Widder captured a deep-sea shrimp defending itself against a predator through bioluminescence.
Due to its truly small size, nanoluciferase enzyme is highly popular, and scientists and engineers worldwide use it for non-invasive imaging of various biological processes. In recent years, luciferases have also found applications in the photodynamic therapy of cancerous diseases.
“However, despite the widespread popularity of nanoluciferase, the molecular mechanism by which this brightest luciferase produces light has not been known until now,” explains the head of the research team, Martin Marek, from the Loschmidt Laboratories of the Faculty of Science at Masaryk University and the International Clinical Research Center of St. Anne’s University Hospital and the Faculty of Medicine of Masaryk University.

Team Leader of Molecular and Structural Biology at Masaryk Univerzity Martin Marek.