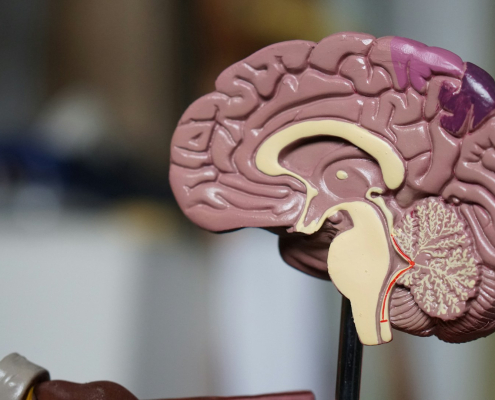
The new CLARA research centre will use artificial intelligence, quantum computing methods, and supercomputers for research of neurodegenerative diseases
Prague, 14 November 2024 – Brain research requires a fundamental change in research approach. Research teams from four Czech research institutes have come up with a completely new direction, teaming up with French and German scientists and…
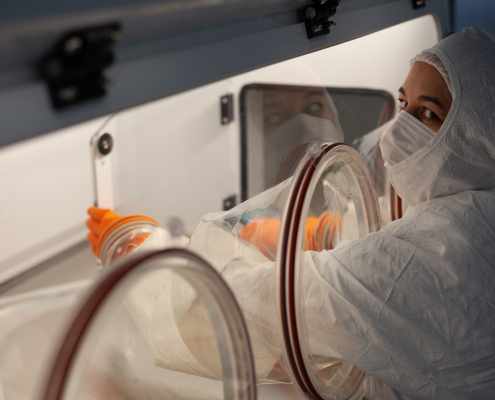
Antiviral cell therapy saves lives where standard treatment fails. It is now covered by insurance.
Cell therapy represents one of the most advanced treatment approaches of our time. It brings back to life patients for whom there has previously been no effective treatment without lasting consequences. Doctors, in collaboration with the team…

Let’s help the sick achieve self-sufficiency. A triathlete from Brno competes against Alzheimer’s and Parkinson’s diseases
Brno native Iva Horčicová is participating in the Triathlon World Championship for the first time this year. The big day will take place on October 17, 2024, in Torremolinos, Spain. Through her race, she aims to support a good cause—research…

