Nanoparticle cancer therapies could reach clinics within a decade, says ICRC researcher Soraia Fernandes
Soraia Fernandes, a Portuguese scientist, has worked as a researcher in the Mechanobiology of Disease Group, led by Giancarlo Forte, for six years. Thanks to the MSCA Fellowships Mobility Support programme at FNUSA-ICRC, she is now undertaking a two-year professional internship at the University of Melbourne in Australia. During her internship, she is studying the potential anti-cancer therapeutic effects of drug-loaded nanoparticles with varying physicochemical properties. We talked about her research, as well as the differences between the scientific environments in Europe and Australia, and the challenges of working as a researcher.

ICRC Researcher Soraia Fernandes
As part of the ICRC, you have the opportunity to undertake a research internship at the University of Melbourne in Australia. How does the scientific environment there differ from what you are used to in Brno and at the ICRC?
There are a few differences. At the ICRC, we have access to a wide range of instrumentation for biological characterisation. Here at the university, our group has its own chemistry laboratories and access to excellent microscopy facilities, including super-resolution microscopes. We can also use other facilities from different departments, which allows us to collaborate with other groups. However, the research environment is essentially the same as in Brno. If I had to compare the work environments, I’d say Australia is slightly more competitive than Europe. I feel that there are more grants and upcoming opportunities in Europe than are available here. Apart from that, I think it’s quite similar. At both the ICRC and the University of Melbourne, I have the opportunity to be part of a international work environment.
What has been the most valuable thing that this fellowship has given you so far?
The possibility to be independent and develop my own idea, as I am responsible for the project and can truly advance that work now. Another valuable aspect is the opportunity to interact closely with two different institutions and build a bridge between the University of Melbourne and the ICRC. This is very useful because the expertise here and in Brno is different, so it helps to combine both. I can use the knowledge from both institutions and apply it to my own project.
“The environment in Australia is more competitive. In Europe, we have more funding opportunities and grant options.”
Your research focuses on nanoparticles and their role in cancer treatment. Could you describe the main purpose of your work?
I began working with cancer-treatment-specific nanoparticles during my PhD. My research focused primarily on solid tumors. While at the ICRC in Brno, I had the opportunity to work on a tumor biology project in collaboration with clinicians at St. Anne’s University Hospital. This gave me the opportunity to develop a deeper understanding of solid tumor biology, particularly with regard to tumor development, growth, and prognosis. And now we know that some characteristics of the tumor can affect the therapeutic efficacy of nanoparticles. For example the excessive extracellular matrix at the tumor site can work as a physical barrier for the penetration and distribution of the nanoparticles within the tumor mass. I apply that knowledge to connect these two fields. I want to study how nanoparticles interact with different components of the tumor microenvironment, including cancer cells, but also other types of cells and non-cellular structures, especially the extracellular matrix. If the extracellular matrix acts as a barrier to the penetration of nanoparticles within the tumor structure, then the effectiveness of the therapy will be reduced. Ideally, the project would allow us to identify features of nanoparticles that enable better tumor penetration and delivery of chemotherapy or other therapies, making them more effective. Additionally, therapies targeting directly the extracellular matrix and their major producers fibroblasts will be studied in order to improve the delivery of the nanoparticles to across the solid tumors.
![]()
![]()
Could you explain the mechanism by which nanoparticles function in the human body? Did you observe any other side effects?
Different types of particles have different mechanisms. At the University of Melbourne, I mainly work with two types of nanoparticles. The first type, which we call glycogen nanoparticles– are natural nanoparticles which are quite biocompatible? This means they are usually not toxic to cells when used in cells and/or mice experiments. The other one is the work of Professor Frank Caruso’s group, which focuses on nanoparticles called metal-phenolic network nanoparticles. Both of these nanoparticles can be used as carriers, allowing us to load them with chemotherapeutic drugs. Nowadays, it is also possible toload different types of nucleic acids, for example for RNA-based therapies. After encapsulating these molecules within the particles, the particles are used to transport them to the tumor sites. This can be done changing the surface properties of the particles to have a little more, let’s say, attraction to certain cells than others. Alternatively, for example, by using specific antibodies that recognize a particular set of cells. Then, once they arrive at or near the tumor, they release their cargo, such as the chemotherapeutic drug, directly at the tumor site, allowing the drug to kill the tumor cells. Ideally, the particles carrying small drugs should be internalised by cancer cells. Once inside the cell, the drug can then take effect and destroy it.
“Ideally, the project would allow us to identify features of nanoparticles that enable better tumor penetration and delivery of chemotherapy or other therapies, making them more effective.”
Nanoparticles are therefore not a replacement for chemotherapy or radiotherapy, but we can say that they serve as an adjunct to treatment, making it more targeted. Does this mean that they reduce the negative impact of aggressive cancer treatments on the patient’s body?
Chemotherapy affects all cells, which is why it causes many adverse effects, such as hair loss, nausea, and vomiting. Patients usually feel quite unwell during chemotherapy because it is a systemic treatment. It’s not targeted. With the nanoparticles, we try to target the therapy to the tissue that needs treatment, thereby avoiding—or at least reducing—many of the side effects that are generally very harmful to patients. Ideally, once optimised, such nanoparticle systems would replace current chemotherapy regimens.
Which method do you use to monitor nanoparticles and evaluate their efficacy?
To track nanoparticles, the easiest way for me is to use fluorescent tags. If we functionalise the surface or interior of the nanoparticles with a fluorescent marker, we can use various microscopy techniques to track their movement within cells or, in the case of in vivo experiments, within mice.
How long do you think it will take for these findings to be translated into medical practice, such as in hospitals or cancer centers?
I’d say maybe a decade or less. I think so, because some of these approaches are already being used in clinics, as for example Doxil, which is a chemotherapeutic drug formulation. In this case, the drug, called doxorubicin, is delivered to the patient in this formulation within a specific type of nanoparticle, a liposome. It can be used as a single treatment or together with other therapies. In addition, nanoparticles are have been used in treating different conditions, such as in COVID vaccines. For example, the newer Pfizer and Moderna vaccines are nanoparticle-based (lipid nanoparticles) and have already been given to most people. So, I don’t think it will take very long to have anti-cancer nanoparticle therapies available and I hope they will be more effective than the options we currently have.
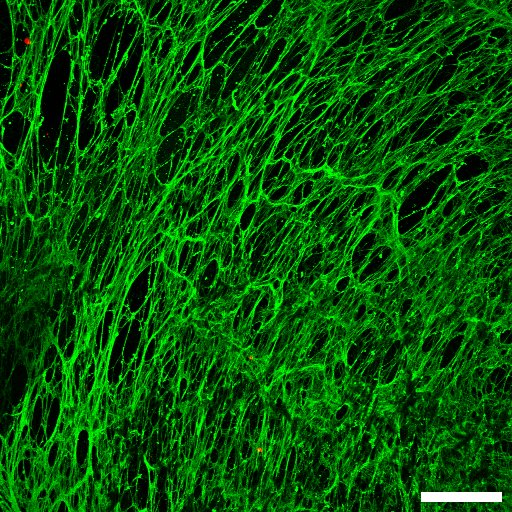
Does your team, or you personally, focus on a specific type of cancer, or on cancer cells in general?
Here in the group where I work at the University of Melbourne, the focus is not only on cancer. The group also tackles other challenges, such as delivering insulin for diabetes or developing other drugs for different pathologies. In my case, I have worked primarily with colorectal, breast and prostate cancer. My work in prostate cancer was mainly developed in Brno, where we developed an organoid model to study cancer progression and collaborated closely with clinicians on the project.
During this two-year internship, what do you hope to accomplish? What is your main goal?
Scientifically speaking, the objective is to describe how the particles interact with the tumor microenvironment and to identify the desirable features we can design into these particles, so that they are not retained in the fibrotic extracellular matrix and can effectively penetrate, allowing the therapy to reach and kill the cancer cells. Alternatively, by modulating the extracellular matrix, we might enhance the ability of drugs to reach cancer cells within the tumor. I have the materials expertise available here in Melbourne, and the organoid models and patient tissues available in Brno. This enables me to test nanoparticles in patient-derived tissues or organoids, which is more interesting and a step forward.
“Cancer treatment enhanced with nanoparticles could mitigate many of the side effects of this otherwise aggressive therapy, such as nausea or hair loss.”
What led you to choose this field of practice? What was your main reason?
I believe that the main motivation for most people working on cancer-related issues is that it is a challenging health problem for which there is still no simple or effective solution, especially once the cancer has spread through metastases. The burden on patients is enormous, and the therapy, with all its side effects, is demanding and not always effective. I unfortunately have experienced the cancer burden in my family. I think most people have. The main goal is to find something better than what is currently available, or at least to understand what isn’t good enough and pave the way for better solutions.
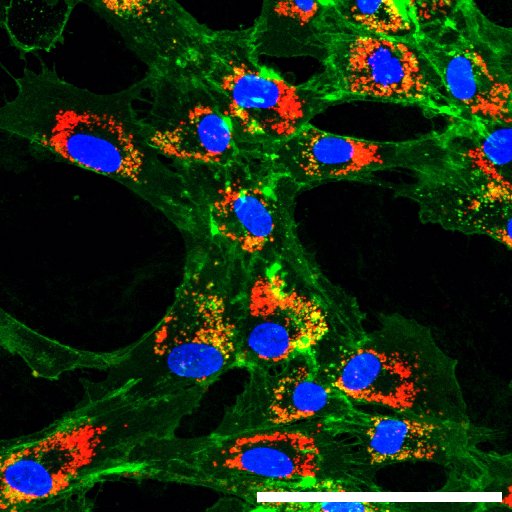
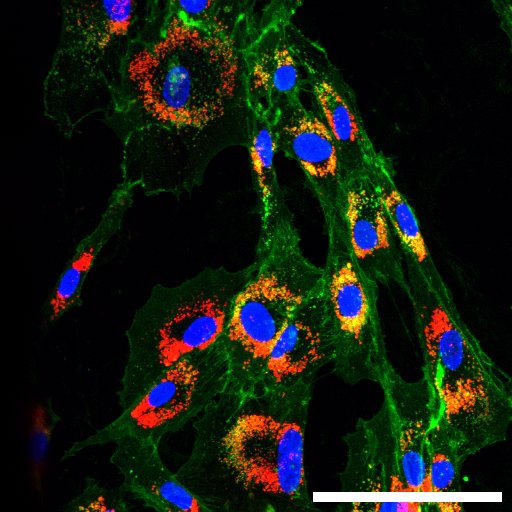
In your view, what is the most challenging aspect of your work or of scientific work in general?
In my field, I think the most difficult aspect is establishing meaningful collaborations with clinicians. Clinicians are not always open to discuss or work closely with scientists at our level. In Brno, it was very good because we had the hospital, and I think people were perhaps more open to exchanging ideas and working together. Connections like this are quite rare. So, yes, I think the hardest part is bridging this gap and collaborating more closely with hospitals and the clinicians who deliver these therapies.
“Connections between scientists and clinicians, like those at ICRC, are rare.”
And what about you personally? Do you see them more as challenges or obstacles?
I’m happy; I like the job. The working hours are sometimes long, but the job also offers flexibility. The opportunity to collaborate with people from around the world and connect with diverse institutions and experts whose knowledge can be pooled is a real advantage. I find that very exciting. However, the lack of stability and work-life balance that this job initially offers could pose a problem for some people. It hasn’t been an issue for me so far, but I understand that some people might see it as an unstable job or one that requires frequent relocation. For me, though, it’s fine. I don’t see it as a drawback — at least not yet!
Do you find it difficult to balance your work commitments with your personal life?
Not really at the moment. It’s just hard being away from my family, but I think it’s an issue that everyone who works abroad faces. Whether or not we want to call it an issue, it also comes with the choices we make along the way. That doesn’t mean it will always be like this, but so far, I’ve managed to strike a good balance between work and my personal life.